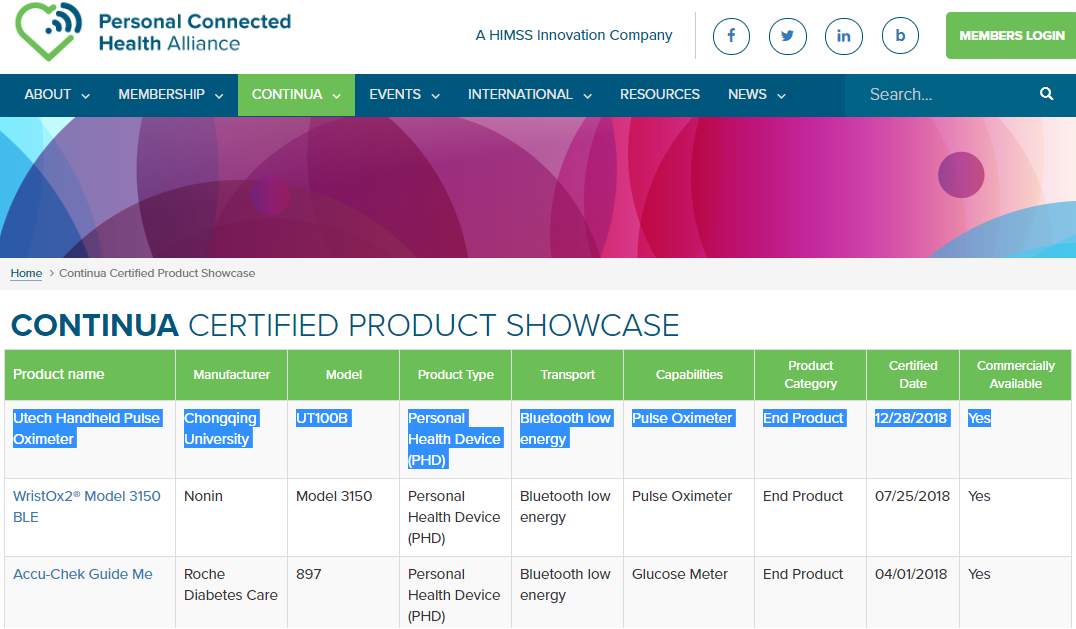
The Project titled “research and development of interop interface for UT100B-type clinical oximeter” directed by Feng Hailing, a postgraduate student of the School of Bioengineering, Chongqing University, has made marked achievements recently. The product has passed the ITU-T H.810 international standard compliance test, and obtained the Continua certification issued by the Personal Connected Heath Alliance. It is the first product in
China
to obtain this certification.
This Project has been substantially supported by Chongqing Municipal Drug Administration, Chongqing Science and Technology Bureau and Chongqing Quality and Technology Supervision Bureau. The marked achievements were the results of industry-university-research cooperation among Chongqing University, UTECH Co., Ltd., and Chongqing Academy of Science and Technology. Before, Continua-certified products were all from developed countries like the
USA
,
Japan
and European countries, such as those manufactured by Intel, Samsung, Philips, Roche Group and IBM. There were no medical apparatus in
China
that had obtained the ITU-T H.810 compliance certification. As such, Chinese products were always behind the competition. UT100B is the first Chinese product to obtain the Continua certification. Furthermore, it also implies that
China
’s medical apparatus manufacturers have mastered the ITU-T H.810 related technologies, as a key step taken towards the international market.
The ITU-T H.810 international standard was released by the International Telecommunication Union run by the United Nations, in an effort to improve the interoperability of the personal connected health area and build an interconnected business environment for the global health industry. This international standard functions as a key element for construction of ICT infrastructures in the electronic health field of different areas, and guarantees safe and reliable transmission of health data between ICT products. It has become an important component of the market admittance system of all mainstream markets in the Europe. The Office of the National Coordinator for Health IT (ONC) and the Food and Drug Administration (FDA) have both included ITU-T H.810 as an officially recognized standard. Manufacturers have been encouraged to implement this international standard by means of fiscal subsidies and appraisal, so as to improve the consistency and safety of interoperability and medical data of medical devices. Furthermore, many EU member states have introduced this standard as a directive standard for construction of their connected health infrastructures. In particular, some of the countries have introduced this standard as a mandatory one to regulate transmission of medical data. Therefore, well-known enterprises in the global IT sector and healthcare sector are now devoted to R&D of medical apparatus in compliance with ITU-T H.810 and acquisition of Continua certification, in an effort to meet the international market admittance requirements and lower the product R&D cost.
The above achievements made by Chongqing University would hopefully boost confidence of domestic enterprises in the connected health area and allow them to have a say in the international market.
Related links: http://www.pchalliance.org/product-showcase
