Recently, the team led by Professor Liao Qiang and Researcher Fu Qian of the School of Energy and Power Engineering of Chongqing University, has published a research paper titled “Hybrid solar-to-methane conversion system with a Faradaic efficiency of up to 96%” in Nano Energy run by Elsevier, in collaboration with Professor Li Yanbo of the University of Electronic Science and Technology of China and Professor Xiao Linzhao from the University of Tokyo. Chongqing University is the first signatory organization. Researcher Fu Qian, Prof. Li Yanbo and Professor Liao Qiang are corresponding co-authors. The paper related experiment has been primarily completed by Researcher Fu Qian and Xiao Shuai, a doctoral student.
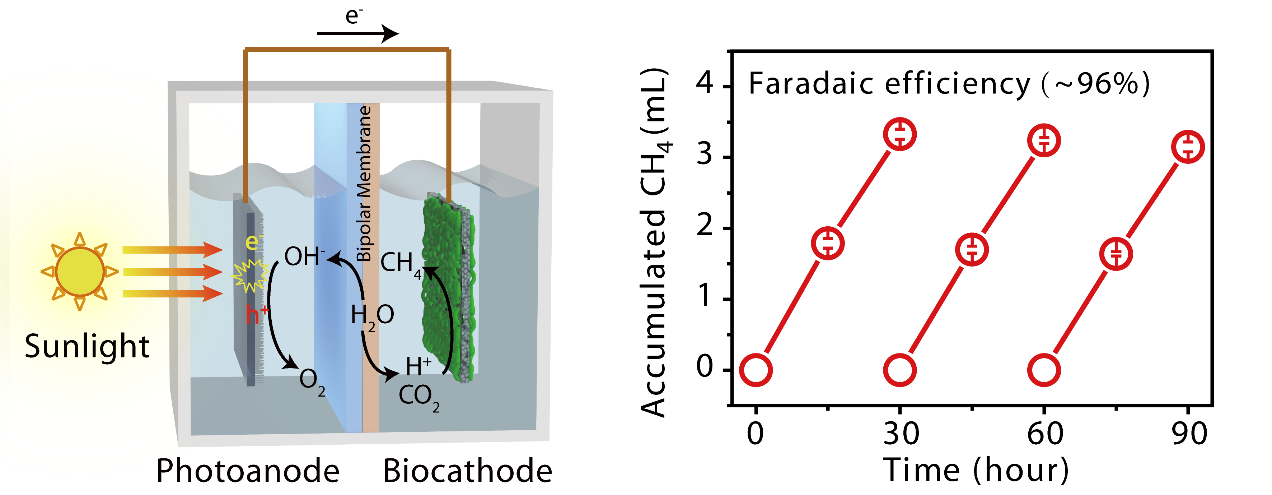
Artificial Photosynthetic Systems simulate the photosynthesis in the natural world and can use solar energy to convert CO2 into chemical fuels and chemicals. It has been a hot research topic in the world in recent more than 10 years. At present, the research of production of chemical fuels with CO2 using artificial photosynthesis mainly focuses on research and development and preparation of catalyst materials with efficient cathode side, low cost and satisfactory stability, and is limited to inorganic catalysts. However, the production of chemical fuels with CO2 through inorganic catalysts reduction implicates problems such as high reaction overpotential, poor product selectivity and low Faradaic efficiency (generally lower than 70%). As a result, the current artificial photosynthesis system requires auxiliary power supply to ensure continued reaction and the later separation of products is highly challenging. By introduction of certain functional microorganisms on the cathode side of the traditional photo-catalytic water splitting system (such as Methanosarcina barkeri), H2 generated on the cathode side can be used to convert CO2 into CH4, isopropanol and other chemical fuels. This is a new way to efficiently reduce CO2 to produce fuels. However, in this “two-way” system, the intermediate product H2 has a low solubility and low mass transfer rate and also requires impressed voltage, thus greatly constraining its development and application.
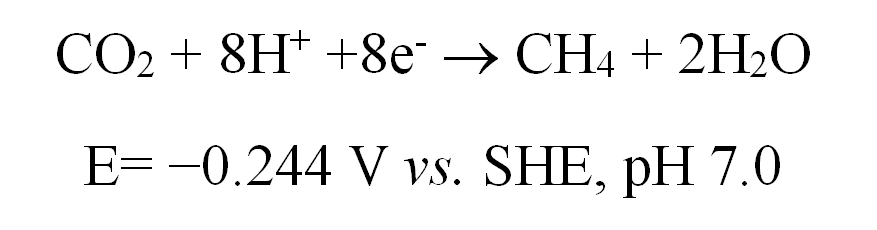
In this paper, the direct electronic transfer between certain microorganisms and electrodes is utilized to build microorganism cathode where “one-step” method can be used to effectively reduce CO2 to generate CH3 (CO2 + 8H+ +8e- ® CH4 + 2H2O, E= −0.244 V vs. SHE, pH 7.0). The reaction process requires no involvement of intermediate product H2. In addition, through coupling of traditional TiO2 photo-anode, an artificial photosynthesis system in which only solar energy is enough to realize efficient reduction of CO2 to produce CH4 without the use of impressed voltage. The overpotential generated from reduction of CO2 to produce CH4 is lower than 50mV, and the product selectivity is extremely high. The Faradaic efficiency of CO2 reduction to produce CH4 is 96%, which is the highest Faradaic efficiency found in domestic and foreign reports. The artificial photosynthesis system combines the inorganic semiconductor that captures sunlight to decompose water with the micro-organic catalyst to reduce CO2, providing a new way of conversion of CO2 into carbon-based chemical fuels.
The research has been supported by the Natural Science Foundation of China and Chongqing Natural Science Foundation.
Link of the paper: https://authors.elsevier.com/a/1Xerm7soS7qCiu
