The research group of Professor Jiang Qihui and Professor Wu Shourong of the School of Bioengineering has recently published an original research paper in Advanced Science (IF: 16.8), an international authoritative academic journal, under the title “Homeostasis Imbalance of YY2 and YY1 Promotes Tumor Growth by Manipulating Ferroptosis”. The issue of the journal was published on May 5, 2022. The first author of the paper is Li Yanjun, a PhD graduate of the School of Bioengineering. Jiang Qihui and Wu Shourong are corresponding authors. Chongqing University is the first completing affiliation and corresponding author affiliation of the paper.
The research group of Jiang Qihui and Wu Shourong revealed the important mechanism of Yin Yang 1 (YY1) and Yin Yang 2 (YY2), which are highly conserved in a variety of species and can regulate 7% of mammalian genes, to jointly regulate the iron death pathway in tumor cells, through the application of molecular biology technologies, such as gene editing and knockout technology, gene silencing technology and gene mutation technology. YY1 and YY2 are both factors of the Yin Yang family (YY family) and feature high homology. This study revealed for the first time that their imbalance promoted the malignant development of tumor cells, which provides an important experimental basis for anti-tumor strategies targeting YY family signal pathways, and has great scientific research significance and potential clinical application value in tumor prevention and treatment.
Yin Yang YY1 is highly conserved in many species and is predicted to regulate about 7% of mammalian genes. YY1 is abnormally highly expressed in tumor cells and promotes tumor occurrence and deterioration by promoting tumor cell cycle process, cell proliferation, angiogenesis, glucose and fat metabolism reprogramming. It is an infamous cancer-promoting gene. Targeting YY1 has always been believed to be a good anti-tumor treatment strategy. However, there has been no successful development of targeted YY1 inhibitors or its application in clinical tumor treatment. It has been found in recent years that another member of YY family, YY2, has low expression in tumor cells, and its high expression has the opposite inhibitory effect on the occurrence and development of tumor. YY2 gene originates from YY1 messenger RNA of reverse transcription transposition, so they are highly homological and can combine with common DNA sequence. However, there have been only a few reports about YY2, and the oncological biological function and role of YY2 in the occurrence and development of tumor are yet to be revealed.
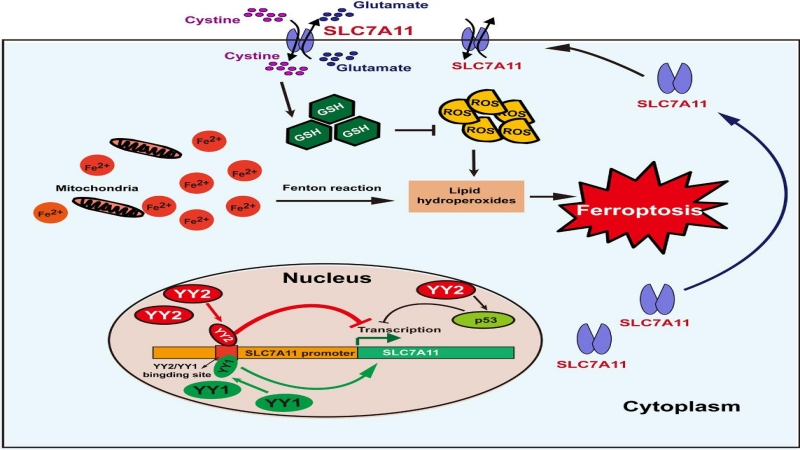
Iron death is an iron-dependent death mode of cells caused by lipid peroxidation found in recent years. Tumor cells promote their own survival rate by improving the intracellular reductant glutathione to reduce lipid peroxidation and inhibiting iron death. As a result, the reduction of iron death facilitates the development and deterioration of tumor. The study showed that YY1 and YY2 could regulate the expression level of SLC7A11 by binding to the promoter of SLC7A11, a key factor of iron death signal pathway, through a shared DNA sequence target. At that time, the binding of YY1 and SLC7A11 promoter promoted its expression level, which resulted in the increase of glutathione content and inhibited the iron death of tumor cells. The binding of YY2 and SLC7A11 promoter inhibited its expression level, reduced the production of glutathione and promoted the iron death of tumor cells. The study revealed for the first time that YY1 and YY2 were very likely to be competitive in binding to SLC7A11 promoter. YY1 has high expression in tumor cells, while YY2 has low expression. Therefore, the findings of the study suggested that the low expression of YY2 in tumor cells would result in the increase of the binding of YY1 and SLC7A11, and the activation of SLC7A11 expression would result in the decrease of iron death in tumor cells, which would promote the occurrence and development of tumor.
The study revealed for the first time that the imbalance of YY family could jointly inhibit iron death signals and promote the occurrence and development of tumors by playing an opposite regulatory role on common targets. The findings revealed an important mechanism by which YY family members regulated common target factors. At the same time, the findings of the study suggested that anti-tumor drugs targeting YY1 need to have high target specificity and avoid affecting its homologous factor YY2 at the same time, so as to offset the tumor inhibitory effect of targeted anti-tumor drugs, which brings up new research idea and lays an experimental theoretical foundation for the successful development of targeted anti-tumor inhibitors. In addition, due to the various roles YY family factors play in regulating biological functions, the importance of balanced regulation of YY family factors revealed in this study may also be of great significance to the prevention and treatment of other serious diseases.
The research group has been focusing on the basic research of oncogenes and tumor suppressor genes in cell cycle disorder and mitotic regulation. It has been engaged in research of YY family for many years, and has successively revealed the role of YY1 in regulating tumor angiogenesis, tumor glucose uptake, tumor pentose phosphate pathway and tumor lipid metabolism, as well as the role of YY2 in regulating tumor suppressor gene p53. In recent years, the research group has made a number of innovative research achievements, and published related research findings in important academic journals such as Science Advances, Cancer Research, EBioMedicine, Oncogene and Theranostics. This research work has been partially supported by the National Natural Science Foundation of China (81872273, 31871367, 32070715 and 82173029) and the Earmarked Funds for Basic Scientific and Frontier Technological Research of Chongqing (cstc2018jcyjax0374 and cstc2018jcyjax0411). Professor Makoto Miyagishi of the National Institute of Advanced Industrial Science and Technology of Japan was involved in part of the technical work of gene silencing technology and gene knockout cell construction.
Paper details: Yanjun Li , Juan Li, Zhuolin Li, Mankun Wei, Hezhao Zhao, Makoto Miyagishi, Shourong Wu*, Vivi Kasim*. Homeostasis Imbalance of YY2 and YY1 Promotes Tumor Growth by Manipulating Ferroptosis. Advanced Science, 2022, May; 9(13):e2104836.
Click to read the full paper: https://onlinelibrary.wiley.com/doi/10.1002/advs.202104836
