Recently, the research group of Lu Yangfan from the School of Materials Science and Engineering, Chongqing University, in collaboration with the research group of Ye Tiannan from Shanghai Jiaotong University and the research group of Hideo Hosono from Tokyo Institute of Technology, published its research findings under a title "Approach to Chemically Durable Nickel and Cobalt Lanthanum-Nitride- d Catalysts for Ammonia Synthesis" in Angewandte Chemie International Edition (factor of influence: 16.823). Chongqing University is the first corresponding affiliation. Associate Professor Lu Yangfan is the first author and the first corresponding author.

At present, Haber-Bosch method commonly is used for industrial ammonia synthesis. However, as the nitrogen molecule has a highly stable N≡N bond (945 kJ·h−1), the reaction usually requires high temperature and high pressure. In recent years, researchers have found that the nitrogen defect of rare earth nitrides has a remarkable ability to activate the N≡N bond. With the use of cheap transition metals (TM) such as Ni or Co, high efficiency ammonia synthesis can be realized at low temperature and low pressure (Nature, 2020, 583, 391). However, most rare earth nitride materials would react with water or oxygen in the air easily, causing material phase transition and leading to catalytic deactivation. This is seriously restricting the extensive application of rare earth nitride catalytic materials.
The research team successfully prepared high concentration Al doped LaN (La-Al-N) through in-situ decomposition of anti perovskite La3AlN, and realized a new type of oxygen resistant, water resistant and efficient ammonia synthesis catalyst. Research shows that the nitrogen defect formed in situ in La-Al-N catalyst plays a critical role in the process of nitrogen adsorption, activation and hydrogenation that produces ammonia. With the use of double active center constructed by cheap transition metals such as Ni and Co, efficient ammonia synthesis (Fig. 1) is realized. Under the conditions of 0.9 MPa and 400 ° C, the ammonia production rate is 5.1 mmol · g − 1·h − 1 and 8.5 mmol · g − 1·h − 1 respectively. The apparent activation energy of the reaction is only~50 kJ· mol − 1, much lower than that of traditional catalysts such as Cs Ru/MgO (~110 kJ · mol − 1).
The highlight of this paper is that through the use of Al doped LaN, the chemical stability of rare earth nitride carrier in air and water is significantly increased. In the water resistance experiment, the author found that La-Al-N could still maintain its original catalytic activity after five cycles of exposure to 34,000 ppm water vapor, while LaN quickly reacted with water when exposed to water vapor and produced La (OH) 3, leading to complete deactivation of the catalyst. DFT calculation shows that Al doping helps increase the stability of La-Al-N surface, and inhibits the adsorption of H2O molecules and the dissociation of O-H bond.
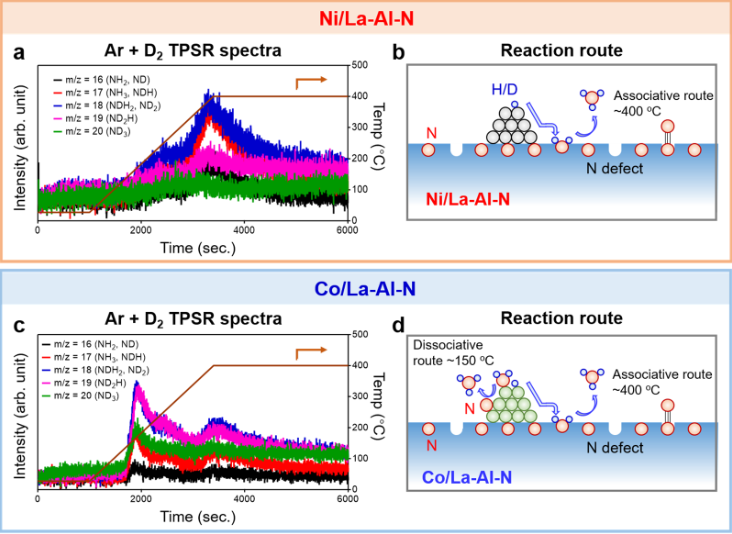
Schematic Diagram of Isotopic Analysis and Reaction Path of Ni/La-Al-N and Co/La-Al-N Reactions
in-situ decomposition of La3AlN was used to realize high concentration Al doping (~25 mol%) in LaN lattice, which further revealed the influence of the formation of La-Al metal bond on the surface free energy of nitride, and significantly increased the oxygen resistance and water resistance of rare earth nitride catalyst. The research findings provide new design ideas for development of ammonia synthesis catalyst materials of high activity and high stability.
Link of the paper: https://onlinelibrary.wiley.com/doi/abs/10.1002/anie.202211759
