Recently, the research group led by Professor Li Yang of the School of Chemistry and Chemical Engineering of Chongqing University has seen dramatic breakthroughs in their research area. Qiu Dachuan and Shi Jiarong (in collaboration), two of the group members, converted the aryne precursor into poly-substitution cyclohexanol acetylene precursor via oxidative dearomatization (as shown in Fig. 1) for the first time. The research findings were published in the latest edition of JACS, the journal of American Chemical Society, under the title “Cyclohexenynone Precursors: Preparation via Oxidative Dearomatization Strategy and Reactivity” (J. Am. Chem. Soc. 2018, 140, 13214–13218) (factor of influence: 14.357). Professor Li Yang started to work at Chongqing University in 2012, and has since then been engaged in research of organic synthetic methodology. Professor Li has been concentrated on the research and application of multi-functionalization of arene by use of aryne, and has achieved a series of research findings in this area in recent years: Chem. Soc. Rev. 2017, 46, 1707; J. Am. Chem. Soc. 2015, 137, 5670; J. Am. Chem. Soc. 2016, 138, 10814; J. Am. Chem. Soc. 2017, 139, 623; J. Am. Chem. Soc.2018, 140, 3555; Org. Lett. 2016, 18, 3130; Org. Lett. 2016, 18, 3726; Org. Lett. 2018, 20, 1919. The latest research progress has allowed the research area of the research group to expand the synthetic benzene ring derivative structure to the construction of compound with the cyclohexane structure.
Fig.1 Preparation of poly-substitution cyclohexanol acetylene precursor via dearomatization of aryne precursor
Structures that contain aliphatic hexatomic rings (also called cyclohexane) are in extensive existence in natural products and medicine molecules, like cholesterol, tetracycline and other bioactive molecules (as shown in Fig.2). Like the aryne chemistry, the aliphatic cyclohexanol acetylene can have a various types of functionalization on the acetylenic bond through reactions such as nucleophilic reaction and cycloaddition. Therefore, the cyclohexanol acetylene can be used as synthesis block to effective construct the poly-substitution aliphatic cyclic hydrocarbons. However, compared with the research of aryne chemistry, the cyclohexanol acetylene chemistry is lagging far behind. One of the reasons is the insufficient development of the reaction types, and the other reason is the difficulty in functionalization of the four non-acetylene bond loci on the cyclohexanol acetylene. To cope with such insufficiencies, the research group of Li Yang tried to prepare the poly-substitution aliphatic cyclohexanol acetylene precursor using the aryne precursor that can easily be functionalized. Document surveys indicate that the oxidative dearomatization can gently and effectively realize the above purposes. The aliphatic hexatomic rings produced using this method may have the original substituent group on the benzene ring reserved, and in addition, it can open the big P bond of the benzene ring to “release” new functional groups.

Fig. 2 Natural products and medicine molecules that contain cyclohexyl
Based on the above assumption, Professor Li’s research group synthesized two types of cyclohexanol acetylene, 1a and 2 (see Fig. 3a), using the oxidative dearomatization method. Compounds 1a and 2 do not only contain carbonyls, methoxy groups, olefin and other substituent groups on their hexatomic rings; those groups may be converted into various types of functional groups. In addition, TMS and OTf groups will not be influenced during conversion. This greatly expands the scope of poly-substitution cyclohexanol acetylene precursors that can be acquired using the dearomatization method (see Fig. 3b). Further researches have revealed that activation of 1a and 2 could effectively produce the related intermediates i and ii of cyclohexanol acetylene. When i and ii interacted with different reagents, they exhibited satisfactory reactivity and selectivity. Some of the reactions include [4+2], [3+2] and [2+2] cycloaddition reactions, nucleophilic reaction and C-N bond insertion (as shown in Fig. 3c). The reaction examples indicate that the two types of reactive intermediates of cyclohexanol acetylene can effectively construct various types of poly-substitution cyclohexanol hydrocarbons, and have huge potential application value.

Fig. 3 Preparation of the two types of cyclohexanol acetylene precursors and research of the reactivity
Through further research of the two types of cyclohexanol acetylene precursors, the research group discovered that there were not only nucleophilic reaction and cycloaddition reaction, the substituent groups allowed them to have some reaction characteristics which the simple cyclohexanol acetylenes do not have. As shown in Fig. 4, under the action of the activating regent, the cyclohexanol acetylene intermediate will interact with the aryl-allyl-sulfoxide and will generate various types of poly-substitution 1, 3-cyclohexanedione. This way, the acetylenic bond was converted into saturated carbon-carbon bond just within one step. This chemical conversion involved allyl- sulfur ylide intermediate v, and the subsequent [2, 3] - ylide rearrangement and cascading. The traditional chemical reaction of benzyne may only produce arene derivatives and cannot damage the big p bond of the benzene ring. This has fully utilized the double-p-bond characteristic of aliphatic acetylenic bonds and used the aliphatic olefins in the subsequent cascade reactions. It revealed the new characteristics of cyclohexanol acetylene chemistry that are different from those of benzyne chemistry.
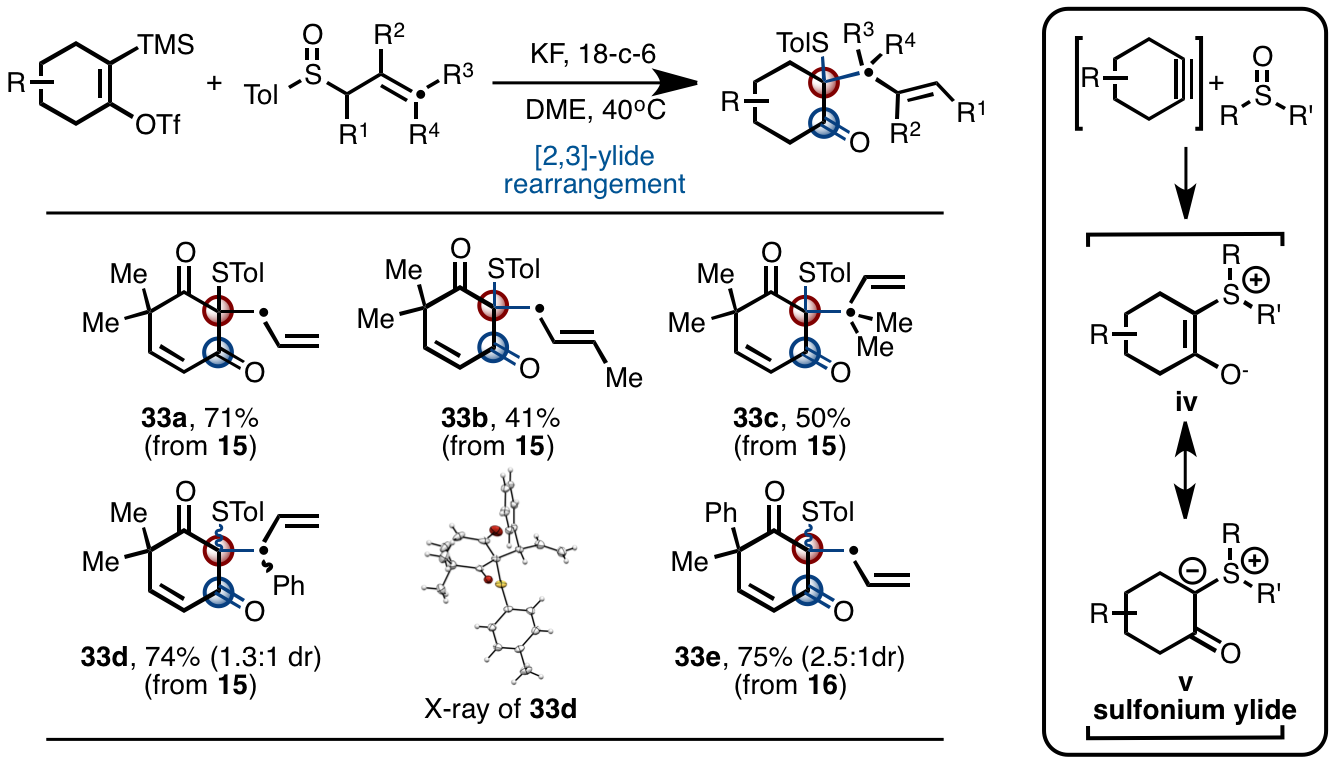
Fig. 4 Reaction between cyclohexanol acetylene precursor and aryl-allyl-sulfoxide
Summary: Professor Li Yang’s research group synthesized two types of cyclohexanol acetylene precursors with poly-functional-group-substitution through oxidative dearomatization for the first time, and managed to solve the problems like difficulty in introduction of substituent group onto the hexatomic ring of traditional cyclohexanol acetylene precursors. Those precursors may go through further derivatization, and have satisfactory reaction activity and selectivity. Besides, they have developed new types of chemical reaction between those novel cyclohexanol acetylene precursors and allyl-sulfoxide. Traditional benzyne chemistry does not have such good reactivity. The research group has provided new research ideas for development of the cyclohexanol acetylene chemistry.
This project has been supported by the general program of the Natural Science Foundation of China and the basic operating funds for scientific researches of Chongqing University allocated by the central government.
Link of the paper: https://pubs.acs.org/doi/10.1021/jacs.8b08915
Website of the research group: http://hgxy.cqu.edu.cn/szll/l_y.htm